ISO 13485 Certification in United States of America
CertEase offers ISO 13485 Certification in United States of America to help medical device manufacturers and related organizations meet the stringent requirements of quality management systems for the medical industry. Our services include a thorough evaluation of your current processes, identification of gaps in compliance, and development of strategies to align with ISO 13485 standards in United States of America. We provide expert guidance to help you implement a structured Quality Management System (QMS), ensuring product safety, regulatory compliance, and consistent quality. With CertEase, you can enhance customer confidence, streamline operational efficiency, and demonstrate your commitment to delivering safe and reliable medical devices.
Looking For Certification?
Have queries?
Ensuring Excellence in Medical Device Manufacturing
In the fast-evolving world of medical devices, ensuring safety, quality, and regulatory compliance is paramount. ISO 13485 certification is the global standard that helps manufacturers meet these requirements while establishing a robust quality management system. Whether you’re a startup or an established manufacturer, especially in cities like New York City, Los Angeles, Chicago, Houston, Phoenix, Philadelphia, San Antonio, achieving ISO 13485 compliance can elevate your operations and reputation.
What is ISO 13485 Certification in United States of America?
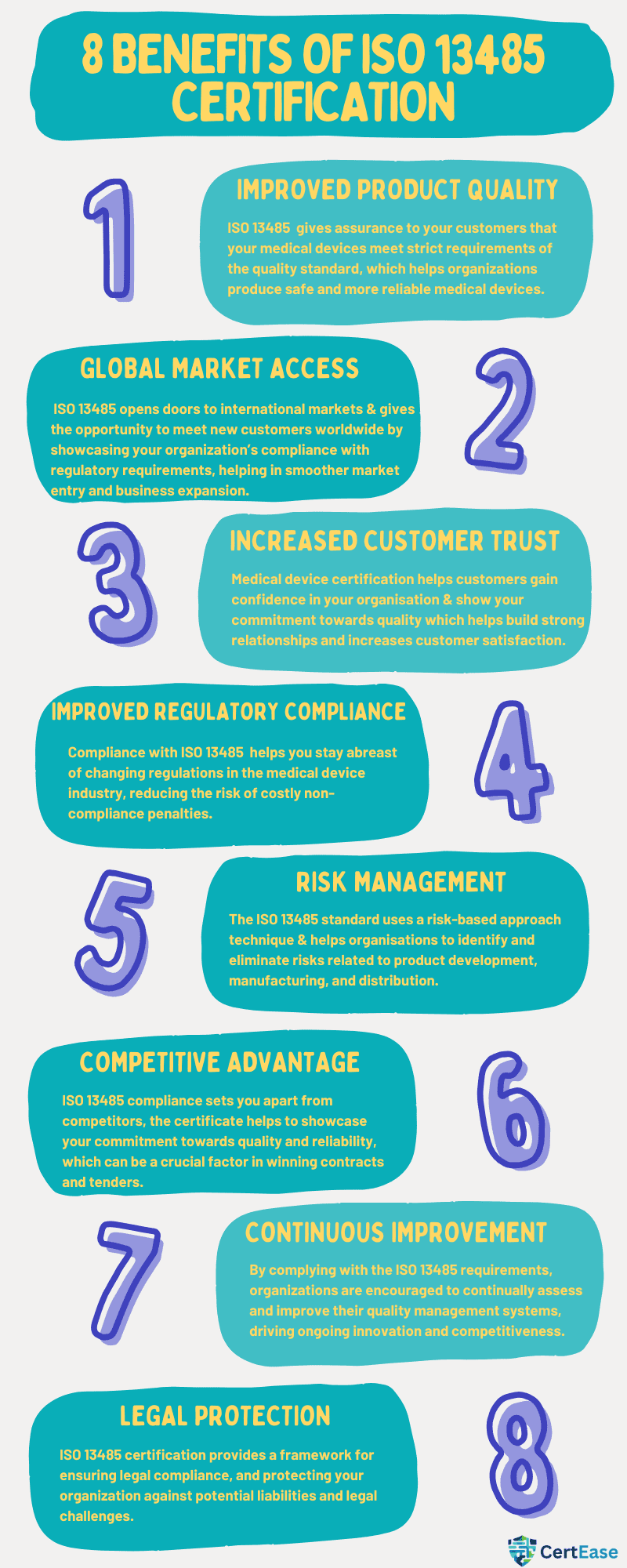
ISO 13485 certification is an internationally recognized standard that ensures an organization’s quality management system (QMS) is designed to consistently meet the requirements of medical device regulations. This certification applies to all stages of a medical device’s lifecycle, including design, development, production, installation, and servicing. Unlike general quality standards in United States of America, ISO 13485 focuses specifically on the stringent demands of the medical device industry, addressing risk management, product traceability, and regulatory compliance. Achieving this certification not only demonstrates your commitment to quality in United States of America but also opens the door to global market opportunities by ensuring your products meet international standards.
Please fill out the details below, and one of our executives will be in touch with you shortly!
Key ISO 13485 Certification Requirements in United States of America
To achieve ISO 13485 compliance in United States of America, organizations must meet specific ISO 13485 certification requirements, including:
- Establishing a quality management system in United States of America.
- Documenting processes for product design and development.
- Managing risks associated with product safety.
- Ensuring traceability of products throughout the supply chain.
- Maintaining effective communication with regulatory authorities in United States of America.
Understanding the ISO 13485 Certification Process in United States of America
The ISO 13485 certification process in United States of America involves several steps:
- Gap Analysis: Evaluate your current processes against the ISO 13485 requirements in United States of America to identify areas needing improvement.
- Implementation: Follow an ISO 13485 implementation guide in United States of America to establish a compliant QMS. This may include training employees, revising documentation, and implementing quality controls.
- Internal Audit: Use an ISO 13485 audit checklist in United States of America to ensure your processes align with the standard.
- Certification Audit: Engage an ISO 13485 certification body in United States of America to assess your compliance.
- Certification: Upon passing the audit, you’ll receive your ISO 13485 certification.
How Much Does ISO 13485 Certification Cost in United States of America?
The ISO 13485 certification cost in United States of America varies based on factors such as company size, scope of certification, and the chosen certification body. While the investment may seem significant, the long-term benefits outweigh the initial expenses.
How Much Does ISO 13485 Certification Cost in United States of America?
The ISO 13485 certification cost in United States of America varies based on factors such as company size, scope of certification, and the chosen certification body. While the investment may seem significant, the long-term benefits outweigh the initial expenses.
Choosing the Right ISO 13485 Certification Body in United States of America
Selecting a reputable ISO 13485 certification body in United States of America is crucial, CertEase can help you choose the right one. Look for accredited organizations with expertise in medical device manufacturing. A reliable partner ensures a smooth certification process and ongoing support.
How to Simplify ISO 13485 Implementation in United States of America?
For many organizations, navigating the complexities of ISO 13485 can be daunting. Hiring ISO 13485 certification consultants in United States of America can provide expert guidance, reducing the time and effort required. Consultants offer tailored solutions, ensuring your QMS meets the necessary standards. Contact CertEase to get an expert Consultant for your requirements.
ISO 13485 Certification Services in United States of America
Professionals like CertEase can assist ISO 13485 certification services in United States of America and provide end-to-end support, from documentation and training to audit preparation and certification. These services are invaluable for companies aiming for quick and efficient compliance.
Achieving ISO 13485 Certification in United States of America
ISO 13485 certification is more than just a regulatory requirement; it’s a testament to your organization’s dedication to quality and safety. By implementing an effective ISO 13485 quality management system in United States of America, you position your company as a trusted player in the medical device industry.
Navigating the path to ISO 13485 certification may seem challenging, but with the right approach and resources, it becomes a manageable and rewarding process. Whether you utilise an ISO 13485 implementation guide in United States of America or work with experienced ISO 13485 certification consultants, especially in cities like New York City, Los Angeles, Chicago, Houston, Phoenix, Philadelphia, San Antonio, achieving compliance ensures your products meet global standards, opening doors to new markets and opportunities.
Ready to take the next step? Partner with trusted us today and ensure your medical devices meet the highest standards of quality and safety.
How CertEase Can Help?
We guide you through every stage of the certification process, from consultation and gap analysis to implementation and certification. Our expert consultants work closely with your team to ensure that your management system is aligned with your business goals and fully compliant with relevant standards.

Start Your Certification Journey Today
Ready to enhance your business operations and achieve compliance? Contact CertEase today to learn how our Management System Certifications can help your organization thrive.

10+
Years of Experience
Why Should You Choose Us?
You’re partnering with a company dedicated to making certification simple, efficient, and effective for your business.
- Expertise Across Industries – With deep knowledge of international standards like ISO, RoHS, CE Marking, and more, we provide tailored solutions for various sectors including healthcare, manufacturing, IT, and services.
- Comprehensive Support – From consultation and training to audits and certification, our end-to-end services ensure a smooth, hassle-free certification process.
- Global Reach – We serve clients worldwide, ensuring compliance with regional and international regulations, no matter where your business operates.
- Proven Track Record – Our team’s success in securing certifications for organizations across the globe speaks to our commitment to quality and client satisfaction.
- Customized Approach – Every business is unique, and we offer personalized services that cater to your specific certification needs, ensuring efficiency and optimal results.
- Ongoing Guidance – Our support doesn’t end with certification; we provide ongoing guidance to help maintain compliance and continuously improve your processes.
Email:
Contact@certease.com
Open Hours:
Mon-Sat: 9am - 6pm
